
(a) F-C 60 view onto (b) F-CNT (c) F-graphene top view (d) F-C 60 view across (e) F-CNT (f) F-graphene side viewįor C-F bonds of fluorinated carbon, the extremely high electronegativity of fluorine endows the C-F bonds varying from ionic bonds, through semi-ionic bonds, to covalent bonds, which results in more electrostatic character in the C-F bonds. The schematic diagrams of some typical fluorinated carbons with an F/C ratio of 1:1. Therefore, it is significantly important to deeply understand the structure for tuning the properties of fluorinated-carbon.
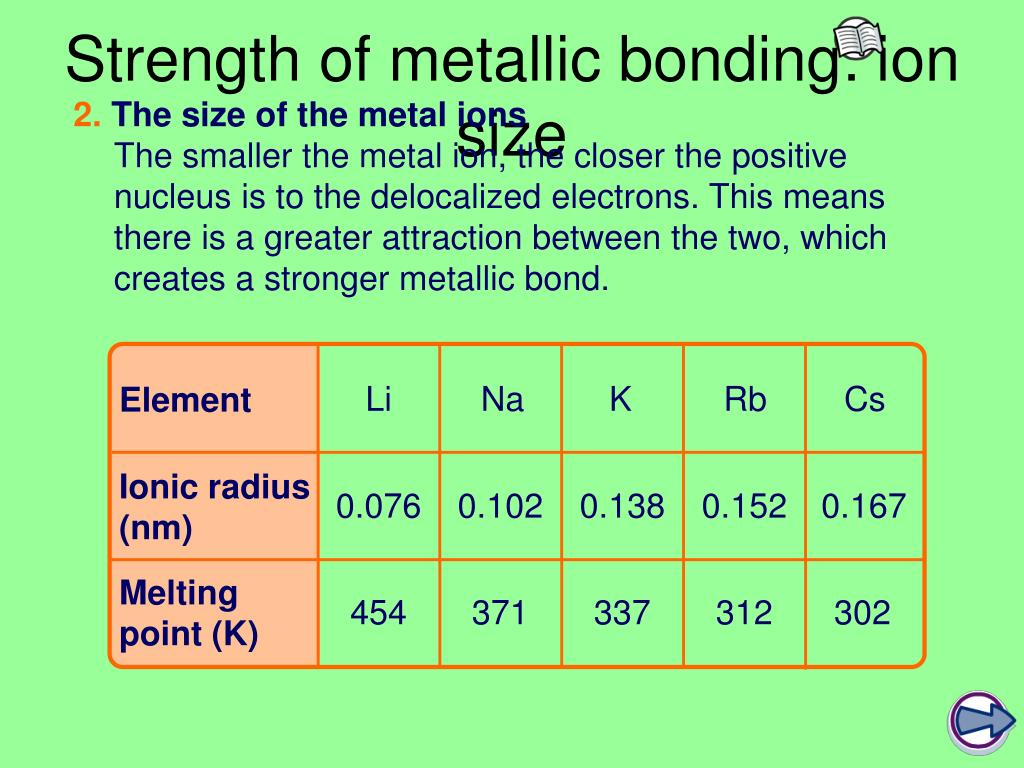
The structure characteristics of fluorinated carbon mainly include C-F bonds and F/C ratios, which can determine the physical/chemical features of materials. Typically, there are three types of fluorinated carbon as shown in Figure 1. 2 Synthesis of fluorinated carbon materials 2.1 Structure of fluorinated carbon Meanwhile, this paper also proposes the potential issues and future opportunities and provides the direction for the advances and applications of fluorinated carbon. In this brief review, we summarize the recent development of structure, properties, selection of carbon source and optimization of fluorination conditions during the preparation and applications of fluorinated carbon. Up to now, great progress has been achieved in the research of fluorinated carbon. Thus, optimizing the composition of C-F bonds and F/C ratios is an efficient approach to obtain fluorinated carbon with high performance. In the case of ultra-high F/C ratio, the rate performance is deteriorated because of the large number of covalent bonds resulting in a poor conductivity. However, the low specific capacity is delivered due to an extremely small F/C ratio when the ionic C-F bond is developed only. For instance, fluorinated carbon is considered as a promising cathode material for high-performance lithium batteries because of its high theoretical energy density of 2180 W h kg −1 and specific capacity of 865 mA h g −1 (for Li/CF 1.0) with a voltage platform of 4.5-5 V for a purely ionic C-F bond. The C-F bonds and F/C ratios have significant effects on the properties of fluorinated carbon materials.

Besides, based on theoretical calculation, the C-F bonds depend on the fluorination levels (F/C ratios). Because the F atom has extremely strong electronegativity, fluorinated carbon involves various C-F bonding characteristics of ionic, semi-ionic, and covalent bonds. The C-F bonds are formed based on the fluorination reactions between carbon materials and fluorinating agents. Thus, the key point of fluorinated carbon materials is mainly about the design of carbon materials and the optimization of fluoridation conditions. The C-F bonds and the F/C ratios deeply depend on the raw carbon materials and fluorination conditions including fluorinating agents, temperature and reaction time. The synthesis methods of fluorinated carbon can be mainly classified into direct gas fluorination, indirect fluorination and plasma-assisted fluorination.

So, fluorinated carbon is a potential material in wide application fields such as self-cleaning, lubricants, super-hydrophobic coating and energy storage. Due to its extraordinary properties, such as good chemical stability, tunable bandgap, good thermal conductivity and stability, and super-hydrophobicity, fluorinated carbon materials attracts more and more attention. The present review will provide a direction for tuning C-F bonding and F/C ratios, developing a safe and efficient fluorination method and popularizing the applications of fluorinated carbon materials.įluorinated carbon (CF x) is a promising member of the carbon derivative family since graphite fluoride was synthesized by Ruff et al. Until now, the potential issues and future opportunities of fluorinated carbon are proposed.
The controllable designing of C-F bonding and F/C ratios play a key role to optimize the properties of fluorinated carbon materials. The fluorinated carbon contains various types of C-F bonds including ionic, semi-ionic and covalent C-F, C-F 2, C-F 3 bonds with tunable F/C ratios. Meanwhile, the applications in energy conversions and storage devices, biomedicines, gas sensors, electronic devices, and microwave absorption devices are also presented. Herein, we present a brief review of the recent development of fluorinated carbon materials in terms of structures, properties and preparation techniques. Fluorinated carbon (CF x), a thriving member of the carbonaceous derivative, possesses various excellent properties of chemically stable, tunable bandgap, good thermal conductivity and stability, and super-hydrophobic due to its unique structures and polar C-F bonding.


 0 kommentar(er)
0 kommentar(er)
